
CDMO服务
CDMO基于医疗器械注册人制度,全力打造医疗器械体外诊断试剂项目委托研发、工艺转化、性能评价与验证、质量体系建设与认证、临床试验、注册申报、产品委托生产、合规化仓储供应链管理等多项服务为一体的体外诊断试剂产品 。
CDMO一站式专业服务平台,为客户提供创新项目研发、临床注册、规模化生产等服务。

CRO
服务为客户提供Ⅱ、Ⅲ类医疗器械体外诊断试剂(IVD)临床试验申请和全球注册(NMPA、FDA、CE、HC、TGA)服务,其中包括,医疗器械临床试验方案设计和咨询,医疗器械数据管理和统计分析、医疗器械临床试验报告编写、医疗器械临床试验监查等。

CSO
作为医药合伙人,专业的市场团队结合国内外市场渠道,以品牌授权和代理协议方式等为客户提供方位的产品市场推广营销及专业的售后技术支持。
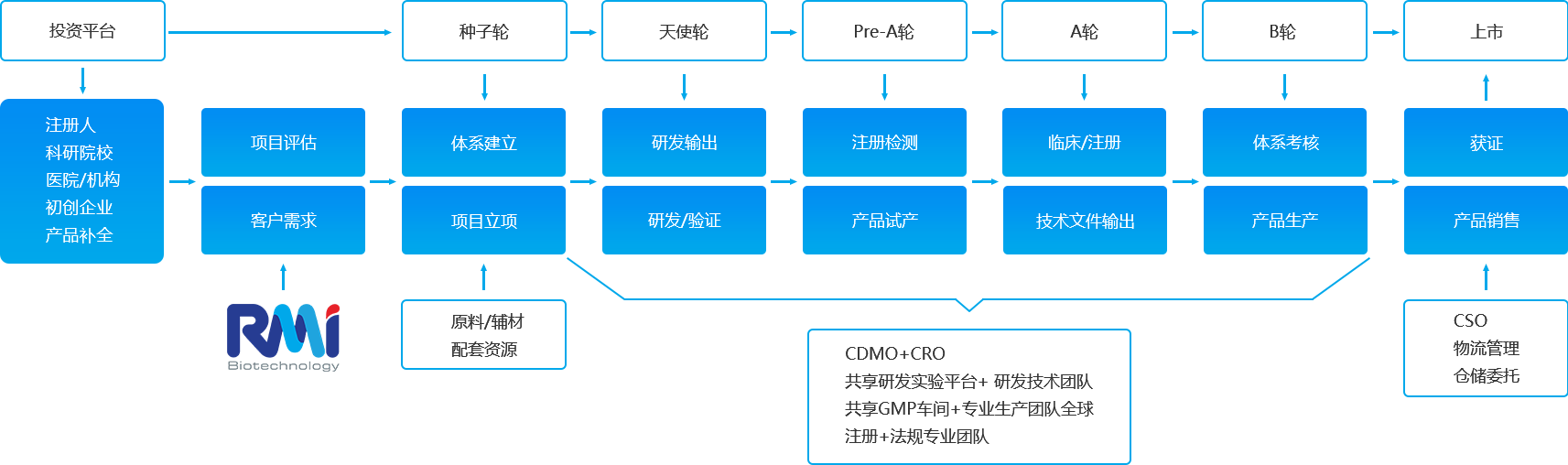

RMi为客户提供项目前期的技术分析、开发路径规划、资源配置、开发周期推算、经济效益测算、风险评估。赋能客户实现研究项目快速产业化,成就创新技术全价值链。

RMi专业团队与客户共同策划研究方案、技术路线、整合资源、风险管理过程管理。

RMi研发团队、质量体系管理团队、法规团队、生产团队、供应链团队、销售团队共10个专业团队按照行业法规(CRO)要求为项目提供专业的一体化解决方案。

RMi共享研发平台和GMP车间全自动智能生产线为项目提供产品委托研发和生产CDMO服务,涵盖体外诊断试剂(荧光层析、胶体金、酶联免疫、POCT、化学发光、分子诊断) 等产品,实现合法合规低投入高效率快速获得许可证,保障优质产品满足市场需求。

战略合作的投资机构和知识产权代理机构资源为项目提供融资和专利申请及增值服务。

RMi国内外市场渠道和专业市场团队,全方位提供产品市场定位、营销推、VI设计、会议会展、产品销售等为企业提供一站式市场推广CSO服务。

专业技术优势

自动化智能生产平台

GMP标准化洁净车间
为了保护客户项目的知识产权,RMi通过切实的、有效的、专业的知识产权保护手段,协助客户积累研发、生产过程中的知识财富和价值。协助客户主自专利的申请,提升项目全链价值。

知识产权保护
全过程闭环数据加密RMi云系统管理,全项目全生命周期模块化设置权限管理,确保项目研发和生产数据安全确保核心技术流失0风险全员依法签订保密协议书和同业竞业协议,保密文件管控,技术资料0泄密风险,客户端自主控制,实时管控项目。

研发和生产数据安全管理
坚守技术创新和高质量为引领的工艺技术,以IVD产业生态链建设和市场导向的有机结合,严格执行法规和ISO13485和GMP质量管理体系,致力打造RMi®品牌产品质量成为行业的标准。

质量体系管理
















































 13632887203
13632887203